Chlorine oxide is a gas used in extremely small amounts to disinfect water. It is a disinfectant similar to bleach that is harmful when used in large quantities. Despite its dangerous nature, this gas plays a significant role in various chemical processes and industrial applications. In the article, we will delve deeper into the properties, applications, and safety considerations related to chlorine oxide.
Introduction to Chlorine Oxide
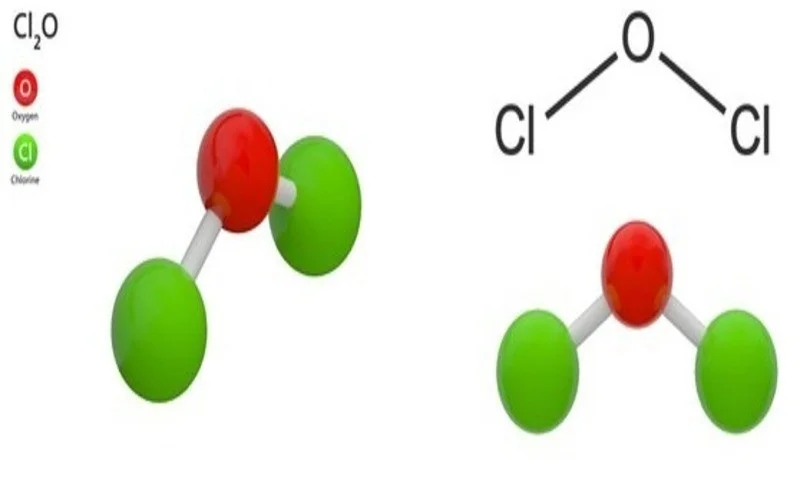
Chlorine oxide, with the chemical formula Cl2O, is a highly reactive and unstable oxide of chlorine. It is a gas with an irritating odor similar to chlorine. It is used to bleach wood pulp, textiles and oils, in processing flour, and for water purification.
As a potent oxidizing agent, Cl2O is capable of oxidizing a wide range of compounds, making it a valuable chemical intermediate.
Chlorine oxide’s unique properties and reactivity stem from its molecular structure, where two chlorine atoms are covalently bonded to a single oxygen atom. This arrangement creates a highly strained and reactive molecule, which readily participates in oxidation-reduction reactions.
Properties of Chlorine Oxide
(Cl2O) is a highly reactive and versatile compound, it has both remarkable physical and chemical characteristics that have garnered significant interest in various fields of study and industrial applications.
Physical properties
- This compound is a reddish-brown gas at room temperature, with an irritating odor, similar to chlorine. It is moderately soluble in water, forming an unstable solution.
- The gas density of Cl2O is 3.09 g/L at 0°C and 1 atm pressure.
- Chlorine oxide’s melting point is -127.6°C (-197.7°F), and its boiling point is 2°C (35.6°F) at atmospheric pressure.
Chemical properties
- This Cl2O compound with a molecular structure of O-Cl-Cl bond molecules bent geometry.
- Compound Cl2O is a potent oxidizing agent due to its strong chlorine-oxygen bond, allowing it to donate oxygen atoms or remove electrons from other compounds readily.
- Cl2O reacts vigorously with organic compounds and can oxidize certain metals, forming metal chlorides or oxides.
- This compound can decompose upon heating or exposure to light. The decomposition process can release chlorine gas (Cl2) and oxygen, or form other chlorine oxides such as chlorine dioxide (ClO2) or chlorine heptoxide (Cl2O7).
Applications of Chlorine Oxide Compound
As a potent oxidizing agent with a unique set of chemical properties, Cl2O finds applications in various industries and scientific disciplines. Here are some key applications of chlorine oxide.
Bleaching and Oxidation Processes of Cl2O
- This compound is utilized as a bleaching agent in the pulp and paper industry, helping to remove lignin and brighten wood pulp.

- It is also used in the oxidation of inorganic compounds, such as the conversion of sulfur dioxide to sulfur trioxide in the production of sulfuric acid.
Water Treatment
This Cl2O chemical is used as a disinfectant and oxidizing agent in water treatment processes, helping to remove contaminants and kill microorganisms. It can be used as an alternative to chlorine or other oxidizing agents in municipal water treatment facilities.

Electrochemical Applications
This Cl2O compound has potential applications in electrochemical processes, such as in the development of advanced batteries and fuel cells, due to its ability to participate in redox reactions.
Environmental Safety
The oxidizing properties of the Cl2O compound make it a candidate for the treatment and removal of environmental contaminants, such as the oxidation of organic pollutants in soil or water.
Precautions when using the chemical Cl2O
Chemical Cl2O is an exceptionally reactive and hazardous compound that necessitates stringent safety measures during handling, storage, and use.
- Wear proper personal protective equipment (PPE) such as respirators, gas-tight suits, gloves, boots, face shields, or goggles chemical-resistant.
- Handle Cl2O chemical in a well-ventilated area, preferably a fume hood or sealed system.
- Use non-sparking tools and grounded equipment to prevent static discharge.
- Implement waste management practices per environmental regulations.
Proper storage and transportation of Cl2O
Adherence to proper storage and transportation guidelines is critical due to the reactive, oxidizing, and potentially explosive nature of the Cl2O compound. Here are some key points about the proper storage and transportation of this compound.
Storage
- Chlorine oxide must be stored in leak-proof, labeled cautions.
- Containers should be kept cool, dry, well-ventilated, and away from incompatible materials like organic compounds, metals, and reducing agents.
- Storage areas should have adequate ventilation, spill containment measures, and easy access to safety equipment.
- Regular inspections should be performed to check for leaks, and container integrity.
Transportation:
- Chlorine oxide is classified as a hazardous material and must follow all applicable transportation regulations.
- It requires proper labeling, placarding, and approved containers/vehicles for shipment.
- Shipments may be limited in size and require special permits depending on the amount.
Summary
The above is some information about the overview of chlorine oxide. Hopefully, the knowledge we share will help readers in applying it in practice. To find out more useful information, please learn more by reading the articles on Dong A Chemical’s website.
Related Articles
Mechanism of Disinfection by Calcium Hypochlorite
Calcium hypochlorite is commonly used as a disinfectant. This disinfectant chemical widely used for ...
What is chlorine dioxide? Address supplier chlorine dioxide quality assurance
Chlorine dioxide is famous for its antibacterial and water treatment and is an extremely powerful ...
Compared Calcium Hypochlorite to Other Chlorine-based Disinfectants
Calcium hypochlorite is one of the most commonly used chlorine-based disinfectants. Chlorine-based ...
What is Chlorine? Properties and Applications of Chlorine Compound in Life
Chlorine is one of the simplest and most common compounds in chemistry. It was discovered in the ...
The Benefits and Best Application of Using Liquid Chlorine for Pool Maintenance
Using liquid chlorine for pools is one of the most effective methods for ensuring the water remains ...
Swimming Pool Water Treatment Chemicals: Ensuring Clean and Safe Water
Proper swimming pool water treatment chemicals ensure they are free from harmful pathogens, algae, ...