Calcium hypochlorite is one of the most commonly used chlorine-based disinfectants. Chlorine-based disinfectants have been widely used for a long time to control and prevent the spread of infectious diseases caused by bacteria, viruses, fungal and other microorganisms. This article delves into the comprehensive understanding of this chemical, covering its physical properties, safety considerations, and diverse applications in water treatment, disinfection, and beyond.
What is Calcium Hypochlorite? An Overview
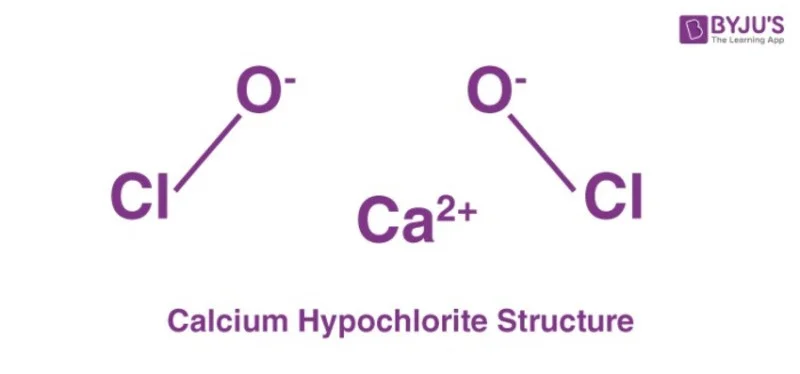
Calcium hypochlorite, with the molecular formula Ca(ClO) 2 , is a widely used chemical compound known for its powerful oxidizing properties and its extensive applications in various industries.
Calcium hypochlorite is produced by the reaction of chlorine gas with calcium hydroxide. It smells strongly of chlorine and dissolves as a solid white powder in water. Ca(ClO) 2 chemicals are widely used for water treatment, disinfecting surfaces, and medical tools.
Properties of Calcium Hypochlorite Chemical
Calcium hypochlorite is known for its unique physical and chemical characteristics, which contribute to its diverse range of applications.
Physical Properties of Ca(ClO) 2
Calcium hypochlorite is known as a white, crystalline powder, it smells strongly of chlorine. This is a chemical compound with the molecular formula Ca(ClO) 2 and a molecular weight of 142.974 g/mol. Its CAS number is 7778-54-3 It has a specific gravity of 1.21 g/cm³, making it a dense material. The solution’s pH ranges from 10 to 12. This compound is known for its powerful oxidizing properties, which enable it to effectively sterilize and disinfect various applications.

Chemical Structure
The structure of Ca(ClO) 2 chemical has been refined using X-ray powder diffraction data. The structure consists of separate layers parallel to the ac plane, where calcium cations are coordinated by chlorite anions, and the oxygen atoms form almost ideal square antiprisms.
Uses of Calcium Hypochlorite
As an antiseptic, calcium hypochlorite offers several advantages, including:
- It is effective against a wide variety of microorganisms, including bacteria, viruses, fungal, and spores.
- Ca(ClO) 2 is extensively used to clean swimming pools, disinfect drinking water, sewage treatment, and water treatment.
- Calcium hypochlorite plays a crucial role in the textile, paper, and detergent industries, leveraging its bleaching and stain-removal capabilities.
- Agriculture and horticulture also benefit from this compound’s disinfecting properties, ensuring the safe treatment of seeds, plant materials, and irrigation water.
In addition, it is highly stable, and when maintained correctly, it can keep its efficiency for a very long time. Ca(ClO) 2 is widely accessible and reasonably priced.
Exploring Calcium Hypochlorite and Other Chlorine Disinfectants
Calcium hypochlorite is one of many chlorine-based disinfectants, each with its unique properties and applications. Additionally, chloramines, chlorine gas, and sodium hypochlorite are the three most widely used chlorine-based disinfectants.
Liquid disinfectant or sodium hypochlorite
It is frequently used for wastewater treatment, swimming pool cleaning, and water treatment. It is affordable and effective against a wide range of bacteria. The effectiveness might be on par with Ca(ClO) 2 chemical.
Chlorine gas
Chlorine gas, which is highly reactive and toxic, is used in industries, swimming pools, and water treatment facilities. This type of chlorine gas is inexpensive and efficient against a wide range of microbes. Extra safety measures must be implemented during handling and transit to guarantee safe handling.

Chloramines
Chloramine, which is commonly used for water treatment and swimming pool cleaning, is formed when chlorine reacts with ammonia. Chloramine is less reactive than chlorine gas, therefore it produces less skin and eye irritation. Chloramine can be used for disinfection, however it is less effective than chlorine gas and may require a longer contact time.
Conclusion
Calcium hypochlorite is a versatile and essential chemical compound with a wide range of applications, from water treatment process to disinfecting drinking water and preventing the growth of algae and other microbes in reservoirs, water tanks, and distribution networks. Its unique properties of calcium hypochlorite make it a crucial player in various industries and applications. As the demand for effective and sustainable disinfection and sanitization solutions continues to grow, the importance of this chemical in maintaining public health and environmental well-being cannot be overstated.
Related Articles
Dong A Chemical – Global Chlorine Supplier
Dong A Chemical - Manufacturer and Global Supplier of Chlorine. Owning a large-scale production ...
The difference between chlorine powder and chlorine pellets in wastewater and swimming pool water treatment
On mentioning kinds of chemicals, chlorine powder and chlorine pellets are the two most popular ...
The Science Behind Chlorine Used in Pools
Swimming pools offer endless fun and relaxation during the warm seasons, but diligent care is ...
What is chlorine dioxide? Address supplier chlorine dioxide quality assurance
Chlorine dioxide is famous for its antibacterial and water treatment and is an extremely powerful ...
Mechanism of Disinfection by Calcium Hypochlorite
Calcium hypochlorite is commonly used as a disinfectant. This disinfectant chemical widely used for ...
What is a Chlorine Oxide Compound? Properties and Applications of Compound in Life
Chlorine oxide is a gas used in extremely small amounts to disinfect water. It is a disinfectant ...