Chlorine is one of the simplest and most common compounds in chemistry. It was discovered in the 18th century and has become an important element in many fields of modern life. In this article, we will learn about the definition, properties and applications of chlorine. From there, we will see the importance and diversity of CLO and its effects on daily life.
What is chlorine?
Here are some of the basic concepts of chlorine as well as the structure of this compound.
Definition
Chlorine is a chemical that exists in gas form, with the chemical formula Cl2. It is a colorless substance with a characteristic odor, heavier than air. It has strong oxidizing properties and has strong effects on many organic and inorganic substances. It has the ability to combine with other elements to form chlorine compounds, for example, hydrochloric acid (HCl) and sodium chloride (NaCl).
Chemical Cl2 is used in many fields. For example, it is used for water treatment, disinfection, as a bleaching agent, and as a major ingredient in the production of other chemical compounds. In addition, it is also used as an oxidizing agent in many industrial and manufacturing processes.
Atoms and structure
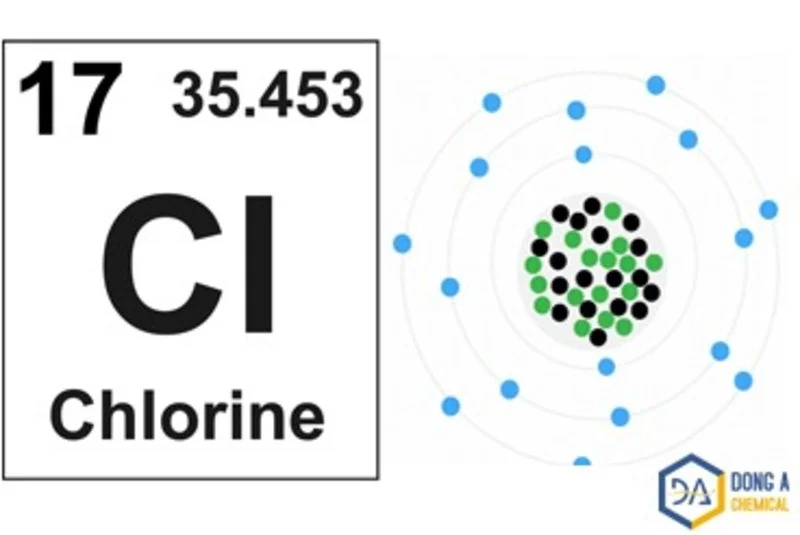
Chlorine has an atomic number of 17, and its molecular structure is Cl2. Each Cl2 atom has 17 electrons, including 2 electrons in the inner shell (K shell) and 7 electrons in the outer shell (L shell). The Cl2 atom has an electron configuration of 1s2 2s2 2p6 3s2 3p5. In the Cl2 molecular structure, two Cl2 atoms of equal atomic mass share a common pair of electrons. These electrons form a single bond between two Cl2 atoms. Therefore, the molecular structure of Cl2 is Cl-Cl, in which the Cl2 atoms are connected through electron interaction bonds.
Properties of Chlorine
Cl2 is a chemical element with the symbol Cl and atomic number 17 in the periodic table. It is a green gas with an unpleasant odor. This compound has many unique properties, here are some key properties of chlorine.
Physical properties
- Chlorine is a yellow-green gas with a pungent odor. It is one of the substances that causes skin burns.
- The boiling point of Cl2 is -34°C and the freezing point is -101°C. This is one of the chemical elements with the lowest boiling point in the periodic table of elements.
- It has a lighter density than air, so it forms a thick layer of gas on the ground, leading to air pollution phenomena.
Chemical properties
- Chlorine is a strong oxidizer, capable of reacting with other substances and causing fires. It also has strong electrostatic properties, so it often creates molecules with the formula Cl2 and easily combines with other atoms such as Na and K to create salts.
- Cl2 compound has the ability to destroy certain bonds in organic molecules, causing oxidation reactions and decomposition of organic substances.
- Chemical Cl2 is widely used in industry as a bleach, disinfectant, and to destroy contaminants in water and air.
What is the application of chlorine in life?
Chlorine can be considered one of the most common substances in our lives. Here are some of the most prominent applications of the Cl2 compound.
CLO in the manufacturing industry
- Chemical manufacturing: Cl2 is used to produce a variety of chemicals, including dioxin, polyvinyl chloride (PVC), chloroform, hydrogen chloride (HCl), hydrochloric acid (HCl), hydrofluoric acid (HF), and other organic substances.
- Iron and steel production: Cl2 is used in iron and steel production to remove impurities and create various metal compounds.
- Food treatment: Cl2 is used to disinfect and preserve food during food production and storage. It can kill pathogenic bacteria and viruses, prolong shelf life and reduce microbial damage to food.
Use Cl2 to treat water and water supply systems
In water treatment, the chemical Cl2 is used to disinfect water and remove contaminants such as bacteria, organic compounds and heavy metal compounds. CLO can effectively destroy pathogens and ensure water is drinking.
In the water supply system, CLO is used to kill bacteria and ensure the supply of clean water to the community. It is often added to water to create a CLO concentration necessary to kill bacteria and pathogens present in the water system.
However, using CLO needs to comply with regulations and instructions on the amount of disinfectant needed, contact time and safety measures to ensure it does not harm human health and the environment.
What are the negative effects of CLO?
As mentioned above, Cl2 is a strong oxidant, capable of causing fire, explosion and toxic effects on human health and the environment. Below are some of the effects and dangers that CLO can cause.
Impact on human health
Short-term exposure to Cl2 can cause sore eyes, sore throat and difficulty breathing. Long-term exposure can cause pneumonia, sore throat, and respiratory problems and lead to decreased lung function. Not only that, it can also cause skin necrosis and serious skin damage if directly exposed. If CLO gets on hair or clothing, it can also cause an explosion when in contact with fire.
Impact on the environment
If CLO leaks into the aquatic environment, it can harm freshwater and marine ecosystems. It has the ability to kill living organisms in water, including bacteria necessary for the water-cleaning process.
Address chlorine supplier, quality assurance in Vietnam
Dong A Chemical Company is a company specializing in providing chemicals in general and chlorine chemicals in particular. With more than 20 years of experience in the industry, we are always committed to ensuring high quality and reliability for our products. The company's professional and experienced technical team always works hard to meet customers’ strict requirements and ensure safety and effectiveness in the use of the company’s chemical products.

Dong A Chemical has helped you answer the question “ What is chlorine ?” through this article. Hopefully, the information in the article will be useful to you. If you need to buy chemicals or treat water with chemicals, don't forget to contact Dong A company via hotline (+84)985797941 or via the official website dongachem.com.
Related Articles
What is a Chlorine Oxide Compound? Properties and Applications of Compound in Life
Chlorine oxide is a gas used in extremely small amounts to disinfect water. It is a disinfectant ...
Mechanism of Disinfection by Calcium Hypochlorite
Calcium hypochlorite is commonly used as a disinfectant. This disinfectant chemical widely used for ...
What is chlorine dioxide? Address supplier chlorine dioxide quality assurance
Chlorine dioxide is famous for its antibacterial and water treatment and is an extremely powerful ...
The Benefits and Best Application of Using Liquid Chlorine for Pool Maintenance
Using liquid chlorine for pools is one of the most effective methods for ensuring the water remains ...
Swimming Pool Water Treatment Chemicals: Ensuring Clean and Safe Water
Proper swimming pool water treatment chemicals ensure they are free from harmful pathogens, algae, ...
How To Clean Stainless Steel with Sodium Hydroxide
Stainless steel is a preferred material for many household items, from kitchen appliances to ...