Water hardness is an important yet often overlooked aspect of water quality. Defined simply, water hardness measures the concentration of dissolved calcium (Ca2+) and magnesium (Mg2+) ions in water. These minerals, along with other trace metals, determine whether water is classified as soft, moderately hard, hard, or very hard. This article explores the definition, causes, impacts, and methods of managing water hardness, providing insights into why this parameter matters for both households and industries.
What is Water Hardness?
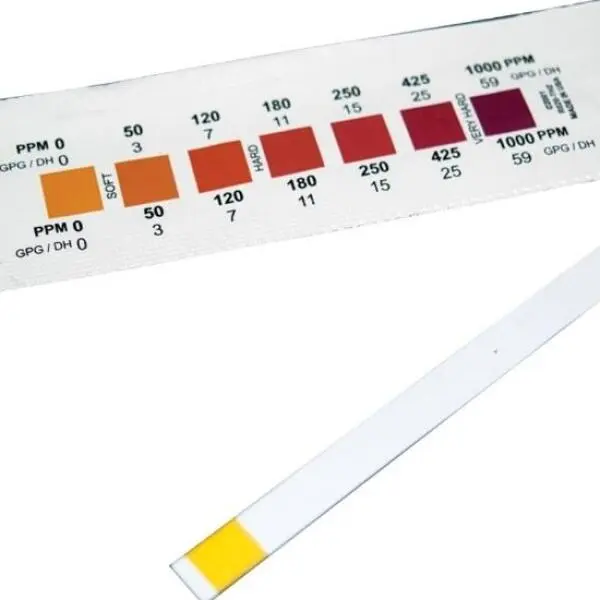
Water hardness is a measure of the amount of dissolved calcium and magnesium in water, typically expressed in terms of calcium carbonate (CaCO3) in milligrams per liter (mg/L). Hard water is characterized by high concentrations of these minerals, whereas soft water has significantly lower levels. General classifications include:
- Soft: 0 to 60 mg/L of CaCO3
- Moderately hard: 61 to 120 mg/L
- Hard: 121 to 180 mg/L
- Very hard: Over 180 mg/L
These classifications help determine water's suitability for various uses, from domestic to industrial applications.
How Does Water Become Hard?
Water hardness originates from intricate geological and chemical processes. As water travels through riverbeds or underground formations, it encounters minerals like limestone (calcium carbonate) and dolomite (calcium magnesium carbonate). These minerals dissolve in water, releasing calcium (Ca2+) and magnesium (Mg2+) ions, which contribute to water hardness.
For instance, in areas with abundant limestone formations, water passing through these rocks absorbs calcium carbonate, increasing its hardness. Similarly, dolomite-rich regions introduce both calcium and magnesium into the water supply. Factors such as temperature and pH also influence the rate at which these minerals dissolve. Warmer water can hold more dissolved minerals, while lower pH levels enhance the solubility of calcium and magnesium.
Well water is particularly prone to hardness due to its prolonged contact with sedimentary layers rich in calcium and magnesium deposits. As rainwater infiltrates the soil and percolates downward, it dissolves these minerals, creating hard water supplies commonly found in regions with specific geological conditions, such as karst landscapes or sedimentary basins.
Understanding these natural processes not only sheds light on why water hardness varies by region but also underscores the importance of local geology in shaping water quality.
Water hardness originates from natural processes. As water flows over riverbeds or through geological formations rich in calcium and magnesium-bearing minerals, these elements dissolve into the water. Well water, in particular, often becomes hard as it percolates through sediment layers containing calcium and magnesium deposits. The result is a mineral-rich water supply that varies by region.

How Does Water Become Hard?
Impacts of Hard Water
Hard water has both benefits and drawbacks, influencing everyday activities and industrial operations.
In Households:
- Cleaning Challenges: Hard water reacts with soap to form "soap scum," a residue that makes it harder to clean effectively. You may notice a film on your hands after washing or spots on dishes after running a dishwasher.
- Damage to Fabrics and Appliances: Minerals in hard water can shorten the lifespan of clothing, leaving them stiff and prone to wear. Similarly, scale buildup in water heaters, coffee makers, and plumbing reduces efficiency and longevity.
- Personal Care: Hard water can leave skin and hair feeling dry and irritated, as the minerals reduce the foaming and cleaning ability of soaps and shampoos.

Hard pool water can clog pipes, damage pool heaters
In Industries:
- Scaling and Efficiency Loss: When hard water is heated, calcium carbonate deposits form scale inside pipes, boilers, and turbines. This reduces water flow, decreases heating efficiency, and increases maintenance costs.
- Corrosion: Ions in hard water can cause galvanic corrosion in metal pipes, leading to leaks and structural damage.
- Product Quality: In manufacturing, hard water can alter the taste, texture, or quality of products, making water hardness control critical.
Benefits of Hard Water
While hard water poses challenges, it also offers some advantages. The minerals in hard water, particularly calcium and magnesium, are essential for human health. According to the World Health Organization (WHO), drinking hard water can contribute to dietary calcium and magnesium intake, especially in populations with marginal consumption of these nutrients.
Water Hardness Control and Management
Given its impacts, measuring and managing water hardness is a priority in many settings. Tools such as titration kits or electronic hardness meters help quantify the concentration of calcium and magnesium ions in water.
Types of Water Softeners:
Several systems are available to address water hardness, each with unique benefits and limitations:
Salt Regeneration Ion Exchange Systems:
- These systems replace calcium and magnesium ions with sodium ions, effectively softening water.
- Maintenance involves adding salt every 2 to 4 weeks, making it a cost-effective and reliable option.
Reverse Osmosis Systems:
- Using a membrane filtration process, these systems remove minerals from water entirely.
- Reverse osmosis requires more maintenance than ion exchange but provides high-quality water for sensitive applications.
Magnetic Systems:
- These systems apply electromagnetic charges to alter water molecules’ behavior, simulating the effects of softened water without removing minerals.
- They require minimal maintenance but may not work in all situations.
Preventing Scale and Corrosion
To mitigate the effects of hard water, regular maintenance and preventative measures are crucial. For example:
- Descaling: Running vinegar or specialized descaling agents through coffee makers and water heaters dissolves mineral deposits, improving efficiency and extending lifespan.
- Pipe Maintenance: Routine cleaning and inspections prevent clogging caused by scale buildup.

Preventing Scale and Corrosion
Conclusion
Water hardness, defined by the concentration of calcium and magnesium ions, significantly impacts household and industrial activities. While hard water can cause issues such as soap scum, scaling, and corrosion, it also provides essential minerals beneficial to human health. By understanding water hardness and employing appropriate softening techniques, individuals and businesses can mitigate its challenges while reaping its benefits. Whether using salt-based systems, reverse osmosis, or electromagnetic methods, addressing water hardness ensures improved efficiency, durability, and quality in both daily life and industrial processes.
For those seeking solutions to water hardness issues, evaluating local water quality and choosing the right softening system is the first step toward maintaining optimal water conditions.
Related Articles
Hardness in Pool: Everything You Need to Know
When it comes to maintaining a swimming pool, keeping your water balanced is essential. One key ...
Top 6 Shrimp Diseases in Aquaculture and Treatment
Shrimp diseases are a significant challenge in the aquaculture industry, affecting shrimp health, ...
What is Hardness in Pond Water and How Does it Affect My Fish?
Understanding the hardness of pond water is vital for maintaining a balanced and thriving aquatic ...
How to Test Pond Water for Optimal Quality
Maintaining a healthy pond ecosystem requires consistent monitoring and management of water quality. ...
Understanding and Managing Nitrite in Pond Water
Nitrite in pond water is a critical topic for pond keepers, especially those with fish such as koi. ...
Nitrite and Nitrate: Key Differences, Benefits, and Risks
Nitrates and nitrites, though closely related, hold diverse roles in our diet and health. From ...