
Nitrates and nitrites, though closely related, hold diverse roles in our diet and health. From supporting cardiovascular wellness to raising cancer concerns, these compounds are ubiquitous in nature, food, and water. Understanding their differences and how they affect us is essential for making informed health decisions.
How are Nitrates and Nitrites defined?
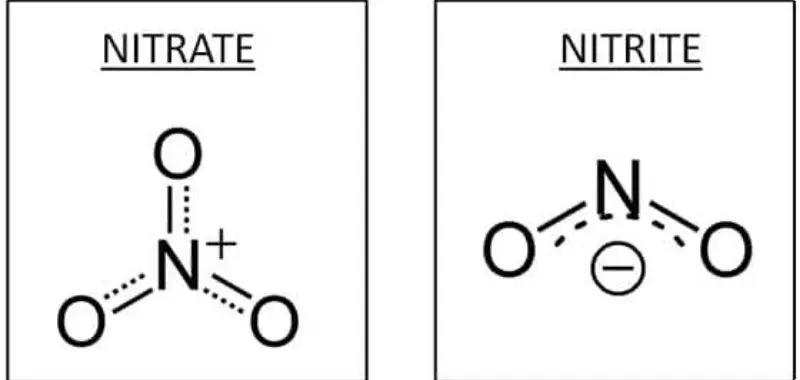
Nitrates and nitrites are two closely related compounds that play significant roles in various biological and industrial processes. Despite their similarities, they differ in composition, structure, and function.
- Nitrates (NO3): Consist of one nitrogen atom bonded to three oxygen atoms. They are relatively stable and inert under normal conditions.
- Nitrites (NO2): Contain one nitrogen atom bonded to two oxygen atoms. They are less stable and can undergo chemical transformations, potentially producing compounds that impact health.
The body can convert nitrates into nitrites through bacterial or enzymatic action. Nitrites, in turn, may form:
- Nitric oxide (NO): Beneficial for cardiovascular health.
- Nitrosamines are substances that have been linked to cancer
How are nitrates and nitrites defined?
The Origins of Nitrates and itrites
Processed Meats Food manufacturers add nitrates and nitrites to cured meats like bacon, sausages, and ham to:
- Prevent bacterial growth.
- Enhance flavor with a salty taste.
- Ensure the meat retains its original color.
However, consuming large amounts of processed meats may increase the risk of digestive tract cancers. The potential risk arises from the formation of nitrosamines during high-temperature cooking.
Vegetables Nitrates are naturally abundant in vegetables such as spinach, lettuce, and beets. Unlike processed meats, vegetable-based nitrates may reduce cancer risk and promote overall health.
- Vegetables contribute approximately 80% of dietary nitrates.
- Nitrates from vegetables can convert to nitric oxide, a molecule essential for cardiovascular function.
Water In some regions, Nitrate leaching from fertilizers contaminates groundwater, leading to health risks, especially for infants. Regulatory agencies monitor nitrate levels in drinking water to mitigate these risks.
Human Body The body naturally produces nitrates, which circulate through saliva, blood, and the digestive system. These compounds exhibit antimicrobial properties, aiding in the elimination of harmful bacteria such as Salmonella.

Nitrate leaching from fertilizers contaminates groundwater
Positive Effects of Nitrates and Nitrites
1. Cardiovascular Health Nitrites can convert to nitric oxide (NO), a molecule that dilates blood vessels and reduces blood pressure. This mechanism underlies the use of nitrate-based medications like nitroglycerin for heart conditions such as angina.
Studies show that consuming nitrate-rich foods like beetroot can lower blood pressure by 4-10 mmHg within hours. Keeping blood pressure under control can help prevent heart disease and stroke.
2. Enhanced Physical Performance Dietary nitrates improve exercise efficiency by increasing mitochondrial function—the energy-producing components of cells. Research highlights the benefits of beetroot juice in reducing oxygen consumption during exercise and enhancing endurance:
- 5.4% reduction in oxygen cost.
- 15% growth in time to exhaustion
- 4% improvement in sprint performance.
Risks of Nitrates and Nitrites
While beneficial in controlled contexts, nitrates and nitrites pose risks when forming nitrosamines, which occur under high-heat cooking conditions.
Nitrosamines:
- Form when nitrites react with amino acids during high-temperature cooking.
- Are carcinogenic and linked to stomach, colon, and kidney cancers.
Processed meats are particularly prone to nitrosamine formation due to their protein content and the presence of sodium nitrite. Cooking vegetables, on the other hand, poses a lower risk, as they generally lack high protein levels and are rarely cooked at extreme temperatures.
Minimizing Nitrosamine Exposure
To reduce exposure to harmful nitrosamines, consider the following strategies:
- Choose nitrate-free products: Look for labels indicating the absence of sodium nitrate (E251) or sodium nitrite (E250).
- Opt for natural preservatives: Some “nitrate-free” products use celery salt, which contains natural nitrates. Give the ingredient list a thorough review.
- Cook at lower temperatures: Avoid high-heat cooking methods such as frying or grilling. Microwave cooking minimizes nitrosamine formation.
- Purchase local products: Support farmers' markets and suppliers offering pasture-raised meat with minimal preservatives.
Comparing Nitrite and Nitrate
Property | Nitrate (NO3) | Nitrite (NO2) |
Composition | 1 nitrogen, 3 oxygen atoms | 1 nitrogen, 2 oxygen atoms |
Oxidation State | +5 | +3 |
Acid Formation | Strong acid (nitric acid) | Weak acid (nitrous acid) |
Molecular Geometry | Trigonal planar | Bent |
Chemical Conversion | Reduced to nitrites | Oxidized to nitrates |
Common Uses | Fertilizers, explosives | Food preservatives |
Additional Considerations
1. Nitrites and Cancer Nitrites can contribute to cancer risk by forming nitrosamines. This is particularly relevant in diets high in processed meats or water contaminated with nitrates.
2. Aquatic Life In aquariums, nitrites are toxic to fish as they bind to hemoglobin, forming methemoglobin, which cannot transport oxygen. This can lead to fish suffocation despite adequate oxygen levels in water.
3. Nitrate Interactions with Medications People using medications for erectile dysfunction (e.g., Viagra) should exercise caution when combining these drugs with nitrates, as the interaction may cause dangerously low blood pressure.
Eliminating Nitrates and Nitrites in Water
Standard water treatment methods like boiling or chemical disinfection do not remove nitrates. Effective techniques include:
- Reverse osmosis
- Ion exchange
- Distillation
Conclusion
Nitrates and nitrites are essential yet complex compounds with both benefits and risks. While nitrates from natural sources like vegetables support cardiovascular health and physical performance, excessive nitrite consumption—especially from processed meats—raises health concerns due to the formation of carcinogenic nitrosamines. By understanding the differences between these compounds and making informed dietary choices, individuals can optimize their health while minimizing risks associated with these substances.
FAQ
Q: What is the primary difference between nitrates and nitrites? A: Nitrates (NO3) have three oxygen atoms, while nitrites (NO2) have two. Nitrates are more stable and often found in vegetables and water, while nitrites are reactive and used as preservatives in processed meats.
Q: Are nitrates from vegetables harmful? A: No, nitrates in vegetables are generally beneficial. They can convert to nitric oxide, which improves cardiovascular health.
Q: How do nitrosamines form, and why are they dangerous? A: Nitrosamines form when nitrites react with amino acids during high-heat cooking. They are carcinogenic and linked to various cancers.
Q: Can nitrates be removed from drinking water? A: Yes, through methods like reverse osmosis, ion exchange, and distillation. Nitrates are not removed through the process of boiling.
Q: Why are nitrites toxic to fish? A: Nitrites bind to fish hemoglobin, forming methemoglobin, which prevents oxygen transport, leading to suffocation even in oxygen-rich water.
Related Articles
Understanding and Managing Nitrite in Pond Water
Nitrite in pond water is a critical topic for pond keepers, especially those with fish such as koi. ...
How to Test Pond Water for Optimal Quality
Maintaining a healthy pond ecosystem requires consistent monitoring and management of water quality. ...
The Science of Water Hardness: How Does Water Hardness Affect My Home?
Water hardness is an important yet often overlooked aspect of water quality. Defined simply, water ...
What is Nitrite? A Comprehensive Guide
Nitrite (chemical formula NO₂⁻) is a compound formed by nitrogen and oxygen, commonly found in soil, ...
Understanding Dissolved Oxygen in Pond Water: Importance, Causes, and Solutions
When it comes to maintaining a healthy pond ecosystem, dissolved oxygen plays a crucial role in ...
Maximizing Carp Production: Is Carp Farming Profitable?
Carp farming, particularly the cultivation of the common carp (Cyprinus carpio), is a cornerstone of ...






