
Understanding the hardness of pond water is vital for maintaining a balanced and thriving aquatic ecosystem. Water hardness impacts everything from fish health to pH stability, making it an essential factor for pond owners to monitor and manage effectively.
What is Carbonate Hardness?
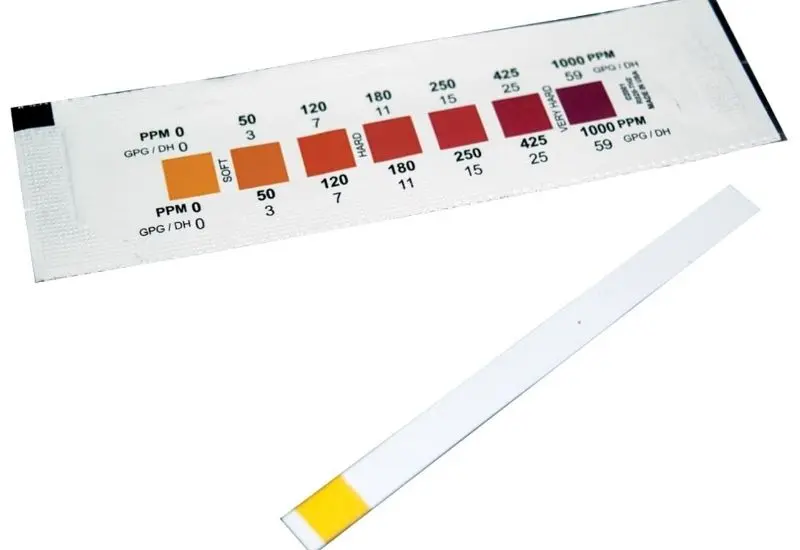
Carbonate hardness, also known as alkalinity, measures the concentration of carbonate and bicarbonate in a pond or lake's water. It is essential for maintaining and controlling water pH levels, ensuring the solution can neutralize acids without drastic pH changes. Alkalinity correlates with the amount of dissolved calcium, magnesium, and other compounds in the water, often increasing in harder water. Over time, alkalinity naturally decreases due to bacterial activity producing acidic compounds that interact with alkalinity components.
In aquatic environments, carbonate hardness plays a critical role in fish safety. For optimal conditions, the carbonate hardness of a pond should ideally range between 50 ppm and 200 ppm. Maintaining this range ensures the water remains conducive to aquatic life.
What is Carbonate Hardness?
Fish-Friendly Environments and Hard Water Benefits
The buffering properties of calcium and magnesium in hard water benefit fish by reducing their sensitivity to acidifying chemicals in treatments like algaecides and herbicides. For instance, in ponds where trout or koi are raised, high carbonate hardness exceeding 50 ppm has been shown to minimize the harmful effects of these substances. In one scenario, a koi pond treated with copper-free algaecides demonstrated significantly improved fish recovery rates compared to those treated with traditional chemicals.
Additionally, aquaculture facilities managing sensitive species like goldfish often report fewer instances of stress and mortality when the water's carbonate hardness is maintained at optimal levels. These benefits are particularly evident in regions where water hardness is naturally high, allowing fish to thrive without requiring extensive chemical adjustments. Regular monitoring and using treatments tailored to high-carbonate environments can further enhance these protective effects.
The buffering properties of calcium and magnesium in hard water benefit fish by reducing their sensitivity to acidifying chemicals in treatments like algaecides and herbicides. Species such as trout, koi, and goldfish are particularly sensitive to these chemicals. High carbonate hardness, exceeding 50 ppm, lessens the impact of these substances, offering additional protection to fish. For these sensitive species, consider using algaecides or herbicides free of copper to avoid potential complications.
GH and KH: Key Components of Water Hardness
General Hardness (GH) refers to the dissolved levels of calcium and magnesium ions, while Carbonate Hardness (KH) measures dissolved carbonates and bicarbonates. These components are vital for fish health and water stability. Natural water sources like streams, rivers, and lakes contain varying levels of dissolved salts, buffers, and nutrients, shaped by geographical and environmental factors.
Aquatic hobbyists can either choose fish suited to local water conditions or adjust the water chemistry to meet specific fish requirements. For instance, in areas with naturally soft water, such as Manchester in the UK, mineral buffers are often added to create suitable environments for fish like koi and tropical species.
pH and Its Relationship with Hardness
pH, GH, and KH are interconnected parameters critical for maintaining healthy aquatic ecosystems. While GH and KH contribute to overall water hardness, pH measures the acidity or alkalinity of the water. A neutral pH is around 7, with values below indicating acidity and above indicating alkalinity.
Maintaining consistent pH levels is crucial for fish health. Rapid pH fluctuations can stress fish, leading to adverse health effects. Most fish thrive in water with a pH range of 6.5 to 7.5. To prevent pH instability, KH acts as a buffer, neutralizing acids like nitric acid produced during the nitrogen cycle. Areas with soft water often require KH supplementation to ensure pH stability.

pH and Its Relationship with Hardness
Osmoregulation and Hardness Levels
Appropriate GH and KH levels support osmoregulation, the process through which fish regulate internal water balance. Fish species like koi carp prefer hard water with high GH levels, which may need periodic supplementation in soft water areas. Conversely, species like South American discus thrive in soft water with low GH levels. Adjusting hardness levels based on species requirements ensures optimal health and environmental compatibility.
For fish requiring soft water in hard water regions, products are available to reduce GH levels. Similarly, soft water enthusiasts can introduce minerals to boost GH and KH as needed, maintaining a balanced aquatic ecosystem.
Interplay Between Water Hardness and pH tability
Water hardness, influenced by dissolved metallic ions like calcium and magnesium, directly impacts pH stability. High KH levels act as a buffer, preventing pH drops caused by hydrogen ions released during fish respiration and digestion. Consistent hardness levels help fish maintain internal salt-water balance, blood pH, kidney function, and reproductive health.
In regions with high KH tap water, regular water changes prevent KH depletion. Conversely, areas with low KH levels require pH buffering substrates like crushed cockle shells to stabilize the environment.
Carbonate Hardness Balancing
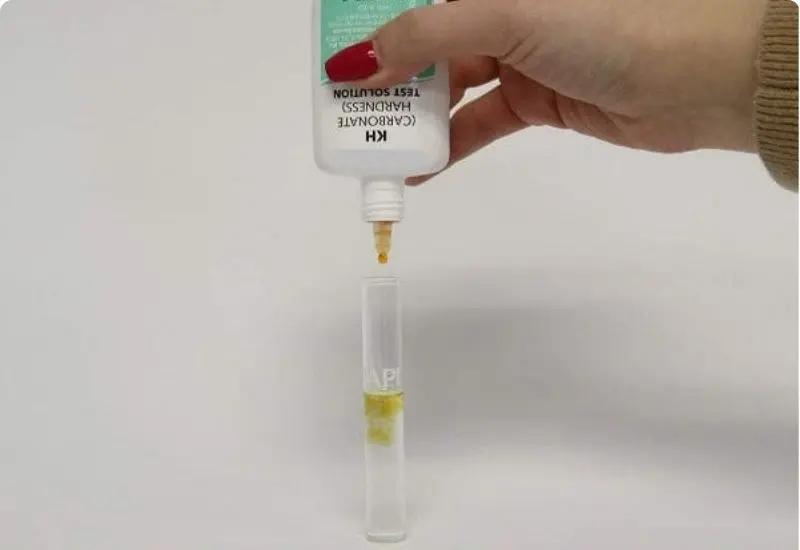
Maintaining the correct KH level is vital for aquatic health. The ideal KH range is between 6 and 8 dKH (107 to 143 ppm). Testing KH involves adding drops of a reagent to a water sample until the color changes, with the number of drops indicating the KH level. For values below 6 dKH, buffering agents such as KH-Buffer Up can be added to restore balance.
To perform a KH test, follow these steps:
- Ensure the test tube is clean.
- Collect a 5 ml water sample.
- Add KH reagent drop by drop, mixing after each addition, until the water changes color from pale blue to yellow.
- Count the drops added to determine the KH level in dKH.
If the KH level is below 1 dKH, immediate action is needed, as such conditions are extremely hazardous for fish.
Carbonate Hardness Balancing
Conclusion
Understanding and managing water hardness in ponds is essential for creating a stable and healthy aquatic environment. By maintaining appropriate GH and KH levels, pond owners can support fish health, ensure pH stability, and replicate natural water conditions. Regular testing and adjustments are key to achieving these goals, safeguarding the delicate balance of aquatic ecosystems.
Related Articles
What is Nitrate and How Can We Manage It in Our Environment?
Nitrate is a vital compound found naturally in our environment and widely used in agriculture, ...
Understanding Nitrate in Drinking Water and Its Impact on Health
Nitrate is a naturally occurring compound that can be found in water, soil, and various foods. While ...
Are Pond Nitrates Dangerous?
High pond nitrate levels are problematic for fish and the overall pond environment. If nitrates are ...
Top 6 Shrimp Diseases in Aquaculture and Treatment
Shrimp diseases are a significant challenge in the aquaculture industry, affecting shrimp health, ...
Hardness in Pool: Everything You Need to Know
When it comes to maintaining a swimming pool, keeping your water balanced is essential. One key ...
The Science of Water Hardness: How Does Water Hardness Affect My Home?
Water hardness is an important yet often overlooked aspect of water quality. Defined simply, water ...






