Nitrate is a vital compound found naturally in our environment and widely used in agriculture, industry, and food production. While essential for plant growth and various industrial processes, its excessive levels can lead to significant health and environmental challenges. Understanding its sources, impacts, and management strategies is crucial for ensuring safety and sustainability.
What is Nitrate?
Nitrate (NO3) is a compound of nitrogen and oxygen that exists naturally in air, soil, water, and certain foods. It plays a vital role in the growth and survival of plants and animals. The human body also produces this compound as part of various biological processes. Widely used in agriculture as a fertilizer due to its high solubility and biodegradability, nitrate also serves in food preservation, pharmaceutical manufacturing, and the production of explosives. To protect against health risks like blue-baby syndrome, the U.S. Environmental Protection Agency (EPA) has set a maximum contaminant level (MCL) of 45 mg/L for nitrate in drinking water.
In organic chemistry, nitrate (NO3-) serves as a functional group with the general formula RONO2, where R represents an organic residue. Most inorganic nitrate salts dissolve easily in water under standard temperature and pressure conditions.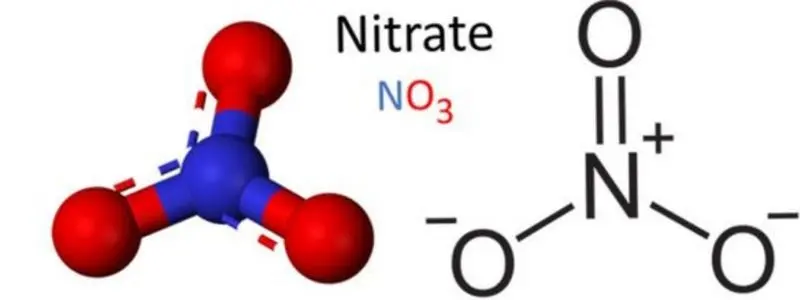
Molecular formation configuration of NO3
Natural Sources of Nitrate
Nitrate originates from various natural and human-related processes. It plays a critical role in the nitrogen cycle, where it forms through nitrification—a microbial process in which bacteria convert ammonia into nitrite (NO2-) and subsequently into nitrate (NO3-). This sequence helps recycle nitrogen in ecosystems, ensuring its availability for plant and animal growth.
In soil and water, microbes decompose organic material and fertilizers, releasing nitrate. Plants absorb this compound to synthesize essential nutrients, with residual amounts often remaining in leaves and fruits. Additionally, many foods and beverages naturally contain nitrate. While usually harmless in moderation, excessive intake or conversion to nitrite can pose health risks. Agricultural activities, particularly the use of nitrate-rich fertilizers, significantly contribute to elevated nitrate levels in groundwater, especially in areas with heavy rainfall or irrigation.
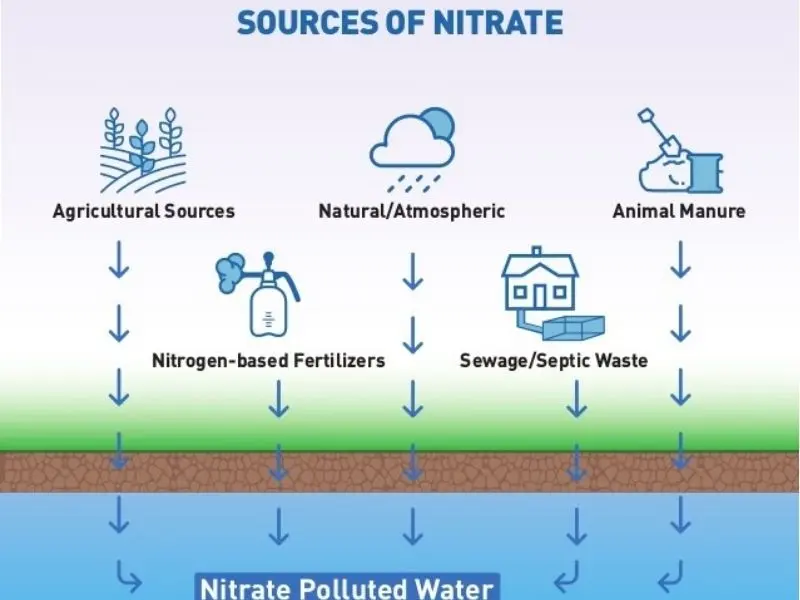
Natural Sources of Nitrate
Nitrate Levels in Groundwater and Potable Water
The concentration of nitrate in groundwater depends on factors such as precipitation, soil composition, and agricultural practices. Research indicates that shallow groundwater often contains nitrate concentrations ranging from 100 to 400 mg/L, while deeper aquifers typically have lower levels of 50 to 100 mg/L. However, the EPA advises keeping nitrate levels in drinking water at or below 45 mg/L to ensure safety.
High nitrate levels in drinking water can lead to methemoglobinemia or blue-baby syndrome, a condition that reduces the blood’s ability to carry oxygen. This can manifest in symptoms such as cyanosis (a blue discoloration of the skin around the mouth, hands, and feet), fatigue, unconsciousness, and seizures in severe cases. Prolonged exposure is also linked to nitrosamines, compounds associated with cancer in animal studies.
Impact on the Environment
Excess nitrate levels in water systems can have profound environmental effects. For aquatic life, high nitrate concentrations can lead to oxygen depletion, causing harm to fish and other organisms. Affected fish may exhibit lethargy, color changes, and even death. When nitrate not absorbed by plants leaches into groundwater, it pollutes drinking water sources and disrupts ecosystems. Furthermore, elevated nitrate levels contribute to eutrophication—a phenomenon where algal blooms deplete oxygen in water bodies, threatening aquatic biodiversity.
Nitrate Detection and Removal
Several methods exist for detecting and removing nitrate from water. Spectrophotometric analysis involves measuring nitrate levels after converting them to nitrite using the Griess reaction. Ammonia, a precursor to nitrate, is also monitored using photometric or potentiometric techniques.
In terms of removal, functionalized chitosan beads have proven effective in laboratory tests. Handheld sensors utilizing molecular modeling allow for real-time water quality analysis, while technological applications such as Android-based apps help monitor nitrate concentrations. These advancements aim to enhance nitrate management and safety.
Looking for Health Advice?
Both the EPA and the Vietnamese National Technical Regulation (QCVN 01:2009/BYT) stipulate maximum allowable nitrate levels in drinking water. These are set at 50 mg/L for nitrate and 3 mg/L for nitrite. To avoid health complications, infants and sensitive populations should not consume water or food containing nitrate levels exceeding these limits.
Nitrate’s Role in Agriculture and Food Safety
Nitrate is a key nutrient for maximizing agricultural yields, but its overuse can lead to environmental and health issues. Farmers are encouraged to use fertilizers judiciously to minimize runoff, monitor soil and water nitrate levels regularly, and adopt sustainable farming practices. These measures ensure balanced crop yields while protecting the environment.

Nitrate is essential for agricultural productivity
Future Research Directions
Future advancements in nitrate management focus on developing mobile apps for real-time monitoring, enhancing adsorbents and filtration systems for efficient removal, and conducting comprehensive studies to assess the long-term impacts of nitrate exposure on human health and ecosystems.
Conclusion
Nitrate is a crucial compound in the natural environment, agriculture, and industry. However, its excessive presence in water and food poses risks to human health, aquatic life, and the environment. By adhering to regulatory standards and adopting innovative detection and mitigation techniques, we can ensure the safe and sustainable use of nitrate in our daily lives.
Related Articles
Understanding Nitrate in Drinking Water and Its Impact on Health
Nitrate is a naturally occurring compound that can be found in water, soil, and various foods. While ...
Are Pond Nitrates Dangerous?
High pond nitrate levels are problematic for fish and the overall pond environment. If nitrates are ...
The Best Pond Water Test Kits for Every Budget
Have you ever questioned the health of your backyard pond? Ensuring the health of your pond is vital ...
What is Hardness in Pond Water and How Does it Affect My Fish?
Understanding the hardness of pond water is vital for maintaining a balanced and thriving aquatic ...
Top 6 Shrimp Diseases in Aquaculture and Treatment
Shrimp diseases are a significant challenge in the aquaculture industry, affecting shrimp health, ...
Hardness in Pool: Everything You Need to Know
When it comes to maintaining a swimming pool, keeping your water balanced is essential. One key ...