
Dissolved oxygen (DO) is a crucial element in aquatic ecosystems, playing a fundamental role in supporting life beneath the water's surface. This article delves into the intricacies of dissolved oxygen, exploring its definition, importance, measurement, and impact on aquatic environments.
What is Dissolved Oxygen?
Dissolved oxygen is the measure of oxygen levels in water.. It's important to note that while water molecules contain oxygen atoms (H2O), this bound oxygen is not what aquatic organisms rely on for survival. Instead, they depend on free oxygen molecules dissolved in the water.
The dissolved oxygen definition encompasses the concentration of non-compound oxygen present in water or other liquids. In limnology, the study of lakes, this is considered the second most essential factor after water itself. The presence of these free O2 molecules in water is vital for the survival of fish, invertebrates, bacteria, and aquatic plants.
Sources of Dissolved Oxygen
Dissolved oxygen enters the water through two main methods:
- Atmospheric Diffusion: Oxygen from the air slowly dissolves into water at the surface. This process is enhanced by wind and wave action, which increases the surface area of water exposed to the air.
- Photosynthesis: Aquatic plants and algae produce oxygen as a byproduct of photosynthesis. This is a significant source of DO, especially in areas with abundant plant life.
Additionally, in some areas, groundwater discharge can contribute to the DO content of streams, particularly in regions where groundwater forms a large portion of streamflow.
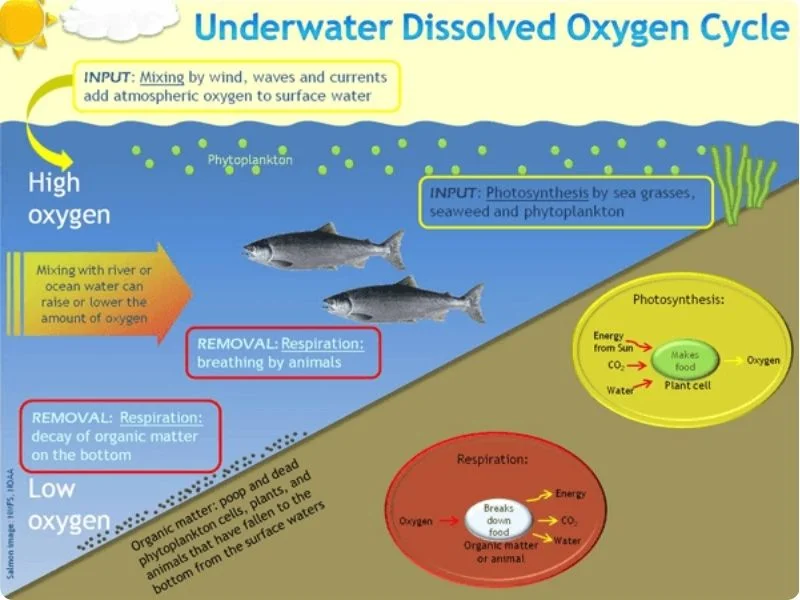
The dissolved oxygen cycle
Why is the Dissolved Oxygen Important?
The importance of dissolved oxygen in aquatic ecosystems cannot be overstated. It is essential for the survival and functioning of aquatic life, from microscopic organisms to large fish. Here are some key reasons why DO is crucial:
- Respiration: Just as terrestrial animals need air to breathe, aquatic organisms require DO for respiration. Fish extract oxygen from water through their gills, while other aquatic life forms have specialized organs for this purpose.
- Ecosystem Health Indicator: DO levels serve as a critical indicator of the overall health of an aquatic ecosystem. Adequate DO levels support a diverse and thriving aquatic community.
- Nutrient Cycling: Proper DO levels are necessary for the efficient breakdown of organic matter and the cycling of nutrients within the ecosystem.
- Water Quality: DO is a key parameter in assessing water quality. Low DO levels can indicate the presence of pollution or an excess of organic matter in the water.
The Importance of the DO Levels
Understanding dissolved oxygen levels is crucial for assessing the health of aquatic environments. Here's a breakdown of significant DO levels and their implications:
- Above 8 mg/L: Ideal conditions for most aquatic life.
- 5-8 mg/L: Suitable for most aquatic species, but some sensitive organisms may be stressed.
- Below 5 mg/L: Stressful for most fish species.
- Below 3 mg/L: Too low to support most fish species.
- Below 1 mg/L: Hypoxic conditions, usually devoid of life.
It's important to note that DO requirements can vary among different species. For instance, cold-water fish like trout and salmon generally require higher DO levels compared to warm-water species.
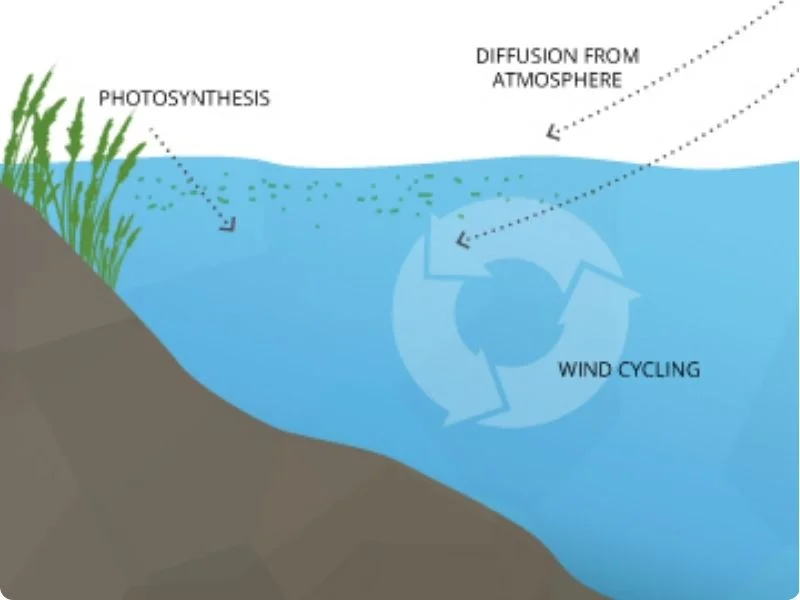
The process of oxygen being dissolved in water.
Causes affecting dissolved oxygen concentration.
Several factors influence the dissolved oxygen concentration in water bodies:
- Temperature: DO levels are higher in cold water compared to warm water. This is why DO levels often fluctuate seasonally, with higher concentrations in winter and lower levels in summer.
- Salinity: Saltwater may contain less DO compared to freshwater under certain conditions. At the same temperature and pressure, seawater holds about 20% less DO than freshwater.
- Atmospheric Pressure: Higher atmospheric pressure allows water to hold more dissolved oxygen.
- Aquatic Life: The presence of aquatic plants can increase DO levels through photosynthesis during daylight hours. Conversely, the respiration of aquatic organisms aorganic matter consume oxygen.
- Water Movement: Rapidly movinnd decomposition of g water, such as in streams and rivers, tends to contain more DO due to increased aeration.
- Pollution: Excess organic matter, often from pollution, can lead to increased microbial activity, depleting oxygen levels.
Measuring Dissolved Oxygen
Accurate measurement of dissolved oxygen is crucial for monitoring water quality and assessing aquatic ecosystem health. DO is typically measured using calibrated water quality probe meters. These modern electronic devices often measure DO alongside other parameters like temperature and pH.
DO can be reported in different units:
- Milligrams per liter (mg/L): The most common unit, equivalent to parts per million (ppm).
- Percent air saturation: Expresses DO as a percentage of the maximum amount of oxygen that can be dissolved at a given temperature, pressure, and salinity.
- Micromoles (μmol): Often used in oceanic studies, where 100 μmol/L equals 2.2 mg/L.
The relationship between mg/L and percent air saturation varies with temperature, pressure, and salinity. To calculate DO concentration from percent air saturation, one can use Henry's Law or refer to oxygen solubility charts.

Measuring DO levels
Consequences of Abnormal DO Levels
Low Dissolved Oxygen (Hypoxia)
When DO levels drop below critical thresholds, it can lead to severe consequences for aquatic ecosystems:
- Fish Kills: Prolonged periods of low DO can result in mass fish mortality events.
- Behavioral Changes: Fish and other aquatic organisms may alter their behavior, moving to areas with higher oxygen levels when possible.
- Reduced Growth and Reproduction: Even if not immediately fatal, low DO can impair the growth and reproductive capabilities of aquatic organisms.
- Dead Zones: In extreme cases, areas of water with little to no dissolved oxygen, known as dead zones, can form. These are particularly common in coastal areas and estuaries where nutrient pollution fuels algal blooms.
Increased Dissolved Oxygen (Supersaturation)
While less common, excessively high DO levels can also be problematic:
- Gas Bubble Disease:Supersaturated water can lead to gas bubble disease in fish and invertebrates. This occurs when dissolved gases come out of solution inside an organism's body, forming bubbles in the bloodstream and tissues.
- Increased Oxidation: Very high DO levels can increase the rate of oxidation of certain substances in the water, potentially affecting water chemistry.
Dissolved Oxygen in Various Aquatic Ecosystems
Lakes and Ponds
In stratified lakes, DO levels can vary significantly with depth:
- Epilimnion (surface layer): Usually well-oxygenated due to atmospheric contact and photosynthesis.
- Metalimnion (middle layer): Can have an oxygen maximum if light penetrates for photosynthesis, or an oxygen minimum in nutrient-rich lakes.
- Hypolimnion (bottom layer): Often has lower DO due to decomposition processes and lack of photosynthesis.
Rivers and Streams
Running water typically maintains higher DO levels due to constant aeration. However, pollution and excessive organic matter can still lead to oxygen depletion in rivers and streams.
Oceans
Oceanic DO levels vary with depth and location:
- Surface waters typically have high levels of dissolved oxygen.
- An oxygen minimum zone often occurs at depths of around 500 meters.
- Deep ocean waters can have varying DO levels depending on circulation patterns and biological activity.

DO Levels in the oceans
Estuaries
Estuarine environments present unique challenges for dissolved oxygen management due to the mixing of fresh and saltwater. Stratification based on salinity can create zones of differing DO concentrations.
Conclusion
Dissolved oxygen is a critical component of aquatic ecosystems, essential for the survival and well-being of aquatic life. Understanding DO levels, their fluctuations, and impacts is crucial for effective water quality management and conservation of aquatic resources. By monitoring and maintaining appropriate DO levels, we can help ensure the health and biodiversity of our water bodies, from small streams to vast oceans. As we face increasing environmental challenges, the role of dissolved oxygen as an indicator of ecosystem health becomes ever more important in our efforts to protect and preserve our precious aquatic environments.
Related Articles
Everything About Ozone Pool Systems Need to Know
Ozone plays an important role in protecting us from the sun, but did you know it can also protect ...
Why Pool Water pH Important and How to Maintain It
Maintaining the correct pool water pH level is crucial for the safety of swimmers, the longevity of ...
Why Is Foam in Pool Water? Causes and Solutions
Contaminants create foam in pool water and tend to stick around, causing your pool an unattractive, ...
pH in Ponds: Maintaining a Healthy Aquatic Ecosystem
When it comes to maintaining a thriving pond ecosystem, one of the most critical factors to consider ...
Pond Filtration 101: Essential Tips for a Thriving Aquatic Environment
Creating a serene pond environment in your backyard can be a rewarding experience. Imagine fish ...
Beyond the Surface: A Deep Dive into Water Parameters
Water quality is a crucial aspect of environmental science, public health, and various industries. ...






